Abstract
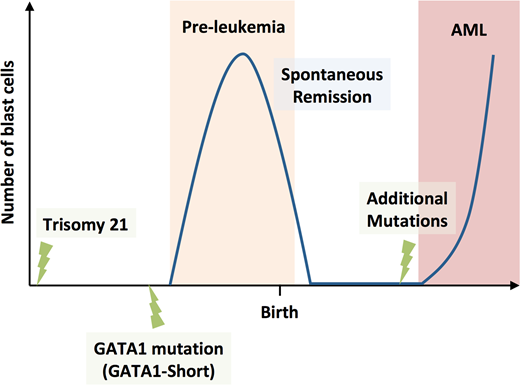
Children with Down syndrome, also known as trisomy 21, have a significantly increased risk of childhood acute leukemia in the first few years after birth. The acute leukemia phase is preceded with a transient pre-leukemia phase in newborns, which is characterized by a clonal proliferation of immature megakaryocytes carrying somatic mutations in the GATA binding protein 1 (GATA1). These acquired GATA1 mutations lead to the expression of the GATA1 short isoform and prevent the expression of the GATA1 long isoform. The pre-leukemia undergoes spontaneous remission within the first few months after birth. In 20% - 30% of the cases, children progress to acute myeloid leukemia (AML) after remission, in which the pre-leukemic clone acquires additional mutations, such as in genes of the cohesin complex. It is hypothesized that this represents a multi-step process of leukemogenesis with three distinct genetic events: trisomy 21, GATA1 mutation and additional tertiary mutations.
Here, we wanted to model the initiation and evolution of Down syndrome associated pre-leukemia and AML by employing CRISPR/Cas9. For this, we developed a CRISPR system that allows the precise manipulation of human hematopoietic stem and progenitor cells using electroporation of Cas9 protein and chemically synthesized gRNAs. We utilized human cord blood and fetal liver as a source of hematopoietic stem and progenitor cells (HSPCs).
We were able to force the re-assignment of GATA1 isoforms to either the short or long isoform using CRISPR/Cas9 in purified hematopoietic stem cells (HSCs), multi-potent progenitors (MPPs), common myeloid progenitor (CMPs) and megakaryocyte/erythrocyte progenitors (MEPs). For each of these populations, we assayed their differentiation potential in single cell in vitro assays. In short, after electroporation and CRISPR/Cas9 mediated re-assignment to either the GATA1 short or long isoform, single cells were deposited onto MS5 stromal cells and were grown for 16-17 days in erythro-myeloid differentiation media. Individual colonies were analyzed by flow cytometry for their differentiation potential and genotyped to confirm CRISPR/Cas9 mediated GATA1 short or long isoform re-assignment. Overall, we were able to observe cell type specific and isoform specific effects on differentiation. For example, re-assignment to the GATA1 short isoform restricted erythroid differentiation and promoted megakaryocytic output in HSCs and MPPs. This effect was both seen when cord blood or fetal liver was used as the source of HSPCs. To confirm the role of the short isoform of GATA1, we transplanted HSCs with GATA1 short in a clonal fashion into immunocompromised mice and after 20 weeks observed grafts with high megakaryocytic output compared to control HSCs. Similarly, GlyA+ erythroid output was significantly decreased compared to transplanted control HSCs.
In summary, this CRISPR/Cas9 system allows us to investigate the differentiation potential of single cells that are restricted to the endogenous expression of either the short or long isoform of GATA1. Future work will include the utilization of trisomy 21 HSCPs and the introduction of tertiary mutations, such as loss of function of STAG2, to potentially progress the model to an acute leukemia phase.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal